Have you ever wondered why the color of a body is that specific color? Like why is the color of the water transparent but the color of the sky blue? Why is the color of an apple red but the color of a banana yellow?
What is color and how does physics define color? Is color just an illusion of our retina diverting the notion of nature?
Why is something black and red but other things white and green? Why does black observe any radiation? Is god involved in all of this, but most importantly, can Physics explain this law of colors? The answer my friend is yes. Physics is involved in all of this and today we are going to learn why a body has a specific color.
What is reflection?
Reflection is a process of emitting photons(or any radiation) when there's an incident of light upon the surface. This is how an object gets color, they reflect light but with a specific wavelength.
For instance, the reflection of the visible spectrum of electromagnetic radiation is why objects have color. A blue material is blue because when all the spectrum of light is incident upon it, the material absorbs almost all incident but emits/reflects the blue color wavelength most frequently and that is why it has a blue color. Similarly, all objects emit different spectra of electromagnetic waves which give their color.
In that sense, we can say that the color of the material depends upon its nature. But how? How does water know to be transparent? What difference does an apple have when it only reflects red instead of any other color?
It's all about molecular and atomic properties:
The reason that each object has a distinct level is because of the internal composition of the molecules in the matter. Every object has distinct energy levels which then interact with different wavelengths of light. Let us go deeper into the atom realm where we can understand what is happening.
1. Energy Levels:
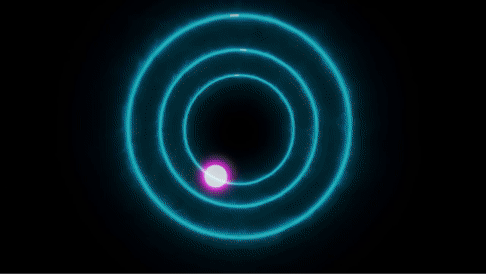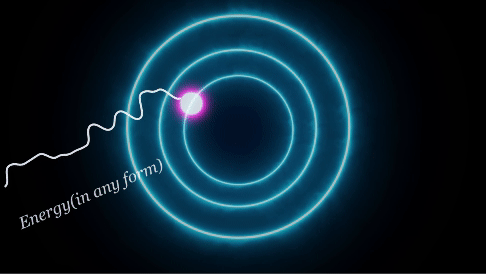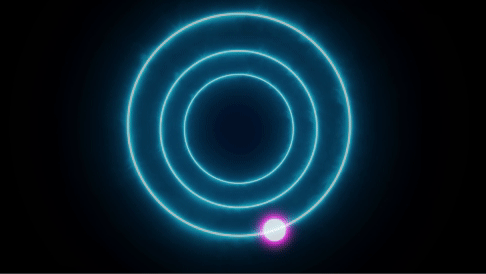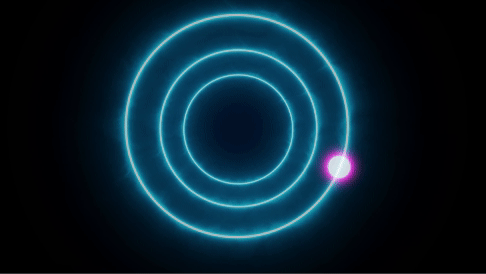
Each electron in an atom can have a specific energy or orbital and every element has different electronic configurations and has different energy levels. Note that these energy levels are quantized and are in discrete form instead of spectrum (why is that, we need to dive in for that). Thus every element has different energy levels - the lowest energy is called the ground state and the highest energy state is called the excited state of an atom.
The electrons not only revolve around their specific orbit but can also jump from one orbit to another. If so, how do we know when this process occurs? Well, when the electrons "jump" between the discrete energy levels, they emit or absorb photons. When an electron absorbs energy, it jumps from a lower energy level to a higher one. Conversely, when it emits energy, it drops from a higher energy level to a lower one. The energy difference between these energy levels determines the energy (wavelength) of the absorbed or emitted photon.
This is a demonstration of how electrons can jump from one state to another...
The same phenomena can also be reversed, with electrons jumping from high to low
3. Absorption and Emission:
Since different atoms have unique distinct energy levels, they can selectively absorb or emit photons with specific wavelengths. When an electron is in an excited state and moves to the ground state it emits a photon with a unique wavelength. This wavelength determines the color of the emission of the body and hence the color of the material.
Electrons when jump from lowest state to highest state---- energy increases--absorption of photons
Electrons when jump from a higher state to a lower--- energy releases--the release of photons.
The emission of photons has a specific wavelength determining the color of the material. Hence the color of the apple is red because the electron configuration of an apple is different. When the electrons of an apple receive energy(light), the electrons transfer to a higher state and after a certain time, they return to their most stable state emitting a photon. This photon corresponds to a red color.
The process of absorption and emission of photons is a key factor in understanding some laws of thermodynamics, for instance, how thermal radiation is affected by temperature. We know that if we heat something for a longer time, it produces radiation. The amount of radiation radiated by the hot object depends upon the temperature i.e. higher the temperature, the higher the radiation radiated, or vice-versa. But, why? How does increasing the temperature affect the intensity of radiation? Well, in this case, the absorption and emission process takes place.
How does increasing temperature increase the intensity of radiation?
When we increase the temperature of the body, we are putting energy into the electrons. Similarly, when the electron absorbs energy, it moves to a higher state. This state is very unstable and according to the laws of mechanics, a body always tries to be in the most stable state, so when it comes down to the lowest state the electron releases some amount of Energy(E). (as we learned from the emission process). If the temperature is very high, many electrons move to the excited state(absorption) and they reach an unstable point from which they go down to their stable state(emission) which releases a huge intensity of photons. The more the temperature, the more this process, and the more radiation it emits. If we increase the temperature substantially, the intensity increases rapidly too just like stars. Since stars have an extremely high temperature from fusion, they release a huge amount of light with highly intense frequency.
Hydrogen Spectrum
The hydrogen spectrum refers to the pattern of spectral lines produced by the emission or absorption of light by hydrogen atoms. This phenomenon is a result of the quantized energy levels of electrons within the hydrogen atom.
When an electron in a hydrogen atom transitions from a higher energy level to a lower one, it releases energy in the form of light. This emitted light is not continuous but consists of discrete lines. Each line corresponds to a specific wavelength or frequency of light, and these lines collectively form the hydrogen spectrum.
The reason that there is a distinct level of light when observing hydrogen or any other elements in the spectral lines is that let's say one electron in the first orbit gets a certain energy and then moves to the second orbital which then releases a certain frequency of electromagnetic radiation. Similarly, if another electron receives a different energy, it moves to a different atom be n=3 or n=4. However, unlike the first scenario, this electron has a different energy and is in a higher state so when it comes down to its lower state it reflects electromagnetic radiation but with a much smaller wavelength. This phenomenon of electrons gaining different energy and maintaining different orbital states make the photons reflected with different color(wavelength) and that is how we observe distinct color or individual spectrum.
This process of emission and absorption of photons is spontaneous and it depends upon the intensity of the radiation. In other words, when we emit light to the blue material, the electrons absorb the light as a form of energy and the electrons transition to the desired states releasing photons and giving the blue color. However, this process continues all the time until and unless there is a light incident on the surface and that is why we see the color blue all the time.
Similarly, if we increase the intensity of the radiation (incident light) we are increasing the energy too. This means that the material is absorbing more energy than before which in turn makes more electron absorbs energy and transition to many levels. As a result, when the incident radiation is more intense, more photons are released from the material and hence the object radiates a much brighter color. If we increase the incident radiation, the reflection can even change the color meaning the blue object might not even reflect blue!
Black Body Radiation:
Now let's try to understand the concept of black-body radiation. Since we have enough info about how radiation is emitted by objects we can now try to understand the black-body spectrum.
Every object in this universe, when light is incident upon it, emits thermal radiation and incident radiation. However, we cannot observe the thermal with our naked eye because it is in the infrared region. We can still use devices like infrared cameras to detect the thermal radiation emitted by the object.
For instance, when we emit light to a blue object, we see the object blue as it reflects two kinds of radiation
1. Incident Radiation: The electromagnetic wave reflected by the blue body that makes it blue.
2. Thermal Radiation: The infrared radiation reflected by the blue body, is a result of thermodynamics.
Why does everybody emit thermal radiation?
Well, since everybody has a positive temperature, there is always a flow of heat/energy in the body. According to the Heisenberg uncertainty principle, a body can never occupy a 0 energy. So a body must have a non-zero energy which corresponds to a non-zero temperature. When a body has temperature, it emits thermal radiation including a black body.
Well, since everybody has a positive temperature, there is always a flow of heat/energy in the body. According to the Heisenberg uncertainty principle, a body can never occupy a 0 energy. So a body must have a non-zero energy which corresponds to a non-zero temperature. When a body has temperature, it emits thermal radiation including a black body.
What exactly is a blackbody?
A black body is an ideal body that absorbs all the incident radiation but only emits the thermal radiation. For instance, when a light is incident upon a body, and it observes all of the incident light but only reflects the thermal radiation(heat) then the body is called a perfect black body.
A black body is an ideal body that absorbs all the incident radiation but only emits the thermal radiation. For instance, when a light is incident upon a body, and it observes all of the incident light but only reflects the thermal radiation(heat) then the body is called a perfect black body.
The Sun can be described as a black body because it absorbs all electromagnetic radiation that falls on it. However, the Sun is not a perfect black body, as it doesn't absorb all of the radiation that falls on it. Its gravitational energy causes it to bend the light around it, but it also emits thermal radiation (heat) to other planets.
Why does a black body absorb all of the incident light?
>>A black body is in thermal equilibrium with the surroundings. This means that the temperature of the black body is always constant making it thermally equilibrium. So when a light is incident upon the black body, the body tries to observe all of the radiation to stay at equilibrium at temperature. When a black body is in thermal equilibrium, it means that it is at a constant temperature, and it is in a balanced state where the energy it absorbs is equal to the energy it emits. This equilibrium is necessary to maintain a stable temperature. In the case of a black body, when it absorbs electromagnetic radiation, its internal energy increases, leading to a rise in temperature. To maintain thermal equilibrium, the black body must also emit radiation. Therefore, to stay thermally equilibrium with the surrounding the black body absorbs all radiation and only emits thermal radiation to follow the rules of thermodynamics.
How to make a blackbody?
We can build a near-ideal black body by allowing a small portion of the light to be reflected.
In the above figure, the light is reflected multiple times around the surface and there is a very low probability of light being reflected outside. However, the object itself can emit thermal radiation since it has heat associated with it. This phenomenon makes that body a "black body". The object absorbs almost all light incident upon it and only reflects thermal radiation.
From this animation, we can see that very little amount of radiation is reflected from the outside of the body. Similarly, since there is the presence of internal energy, from the law of thermodynamics, it should emit thermal radiation as well. Thus, this object emits thermal radiation while observing incident radiation causing it to create a perfect black body.
Why are black holes, not black bodies?
Let's consider black holes for a minute. Black holes have an extremely strong gravitational force that pulls all of the objects including light. Similarly, according to Hawking's radiation, a black hole also emits Hawking radiation by absorbing one particle and leaving another. Then shouldn't it be considered a black body? In other words, if a black hole demonstrates all the properties of an ideal black body, is it not considered a black body?
Well, a black hole is not a black body...
>>> We have to understand that the properties of black body radiation solely depend upon the temperature. It is the consequence of the law of thermodynamics. The black body tries to stay in the thermal equilibrium and when electromagnetic waves are incident upon it, the body might not be in the thermal equilibrium so instead it absorbs the radiation. Now since it absorbs the radiation its energy increases.
This is where the black body becomes different from ordinary matter. The ordinary matter absorbs the radiation and the electron's energy increases completing the ground state to excited state cycle which in turn reflects the color of the material and some part of the radiation. However, a black body absorbs the radiation and since it always stays in the thermal equilibrium, it reflects the incident radiation as a form of thermal radiation (heat) to balance its energy. The process of absorption and emission continues until the black body reaches a state of thermal equilibrium, where the rate of absorption equals the rate of emission. At this point, the black body maintains a constant temperature.
Despite having almost similar properties between a black hole and a black body, there is still a major difference between these two. A black body absorbs and emits radiation because of its temperature. A black body absorbs radiation because of the gravitational force, an effect of the space-time bend. The effects of black holes are due to the quantum nature of particles, and anti-particles and don't depend upon the temperature of the body. So, while both black holes and black bodies can absorb radiation, the mechanisms and underlying principles are different. Black holes don't fit the classical definition of a black body because they lack a traditional surface, and their emission is a consequence of quantum effects near the event horizon rather than the thermal properties of a material.
Why is the Sun considered a black body but black holes aren't?
If black holes are not supposed to be black bodies because of their distinct properties in comparison to black bodies, then why is the Sun considered to be a perfect example of a black body. The Sun has almost the same properties as a black hole but with less gravity. The Sun absorbs all the incident radiation because of its gravity and it radiates huge amounts of energy because of its high temperature.
This is where black holes differ from the Sun. The Sun shows the property of the ideal black body. When the temperature of the Sun is very high, it radiates thermal radiation in this case electromagnetic waves, in the thermal state. However, a black hole doesn't depend upon the thermal equilibrium state. In other words, the Sun radiation depends upon the temperature just like how a black body does.
Let us now try to understand the relation of temperature with the black body.
Wien's Displacement Law:
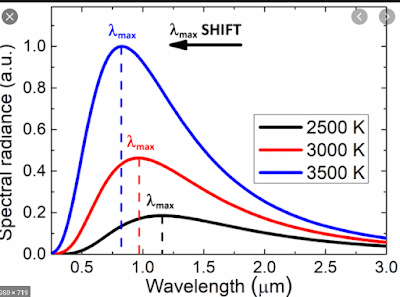
When scientists observed the relation between the intensity of the electromagnetic wave's spectral energy and the object's wavelength (in this case, black bodies), they found a pattern in the graph that shows the relationship between these two.
In this graph, we see that the intensity of the radiation increases as the wavelength gradually increases. But when the wavelength is at a specific point (λ), the intensity is at maximum. This point where the intensity of the radiation is maximum corresponds to a wavelength which is called λmax.
Note that this figure is only for a body that has a certain constant temperature, T1. Now let us try to add more temperatures to the same body; T1 and T2.
When the temperature increases (T1<T2<T3), λmax shifts towards shorter wavelength. This means that when we increase the temperature of the body, it emits wavelength at a very small size. For example, a body that emits 800nm at T1 temperature emits less than 800nm at T2 temperature (assuming T2 is greater than T1). In other words, temperature is inverse to wavelength.
The higher the temperature of the body, the smaller its wavelength radiated...
This graph is proved experimentally and the pattern/graph from this experiment was one of the biggest breakthroughs in physics back in the early century. Similarly, a law was derived from this graph called Wien's displacement law which states that the product of the wavelength of the black body and its corresponding temperature is always a constant. In other words,
λ1*T1=λ2*T2=λ3*T3=.........λn*Tn= b (constant)
Thus, λ(max) * T = b
where b is called the 'Wien's constant' and its value is 2.898*10^-3 mk.
This numerical value for b only works for the black body.
By looking at the black body spectrum, if we know the λmax, we can find its corresponding temperature (T) without even measuring the body's actual temperature.
Why is Wien's displacement important?
This law is one of the most important laws in thermodynamics and one of the reasons behind it is the result of the temperature of the Sun. As we already discussed, the Sun can be considered a black body since it absorbs incident radiation and emits waves as a result of its high temperature. This means that we can use Wien's formula to calculate the temperature of the Sun. For that, we need to find the λmax (wavelength where the intensity is max) of the Sun's light.
Similarly, we can also find the temperature of any stars visible from the Earth's surface by just looking at the spectrum of the light radiated by the stars. This notion of determining the temperature of a black body without actually measuring the temperature still plays a huge role in astronomy.
Stefan's-Boltzmann Law:
If we try to find the area under the graph as shown in the figure, we can get the total intensity at that temperature. Let us try to add more temperature in this graph to see the relation between the intensity and the temperature of the black body.
From here, we can clearly see that the higher the temperature of the black body, the more intense radiation is emitted from the black body.
Therefore, the area under the graph = total intensity radiation at that temperature ∝ Temperature (T)^4
(proved experimentally)
Since intensity = (Q)/(A*t)
We can say that (Q)/(A*t) ∝ T^4
Since we know that the temperature of the body depends upon the wavelength and from Wien's displacement law, we know the inverse relation between temperature and wavelength.
Here, λ1(max) is smaller than λ2(max) and λ3(max), we can say that T1 is greater than any of the other bodies.
Therefore, T1>T2>T3 (without even actually measuring the temperature of the body).
Nishant Chaudhari
Tags:
Black body radiation











it's legend...waitforit.....dary.
Btw awsome blog man. Hope to meet u in person some day.
Thanks a lot!
Dictionary is not enough to describe your content, beyond this universe ❤️
Your comment means a lot! Thank you so much!!